
Defending the Human Intestinal Microbiome With Artificial Biology
An engineered reside biotherapeutic protects the intestinal microbiome in opposition to undesirable penalties of antibiotic therapies and could possibly be developed as a easy and efficient co-therapy.
Many people have skilled our intestine thrown off-balance on account of an unavoidable antibiotic remedy. Antibiotics not solely kill the pathogenic micro organism inflicting an an infection, additionally they indiscriminately wreak havoc on the trillions of “good” micro organism making up the human microbiota. Often known as “dysbiosis,” this alteration of our intestine microbial composition triggers discomforting diarrhea in as much as 35% of sufferers within the brief time period, and might take months to resolve, usually with the assistance of dietary corrections and dietary supplements. In some sufferers, the microbiota may even be completely disturbed, which turns into a severe threat issue for a number of autoimmune, metabolic, and neurological illnesses.
It's because now we have developed a mutually extremely useful relationship with the microbes in our intestine microbiome and in reality fashioned a “superorganism” with them during which we as “hosts” form our microbiome’s composition with our intestine setting, in addition to our consuming and different habits. In flip, our microbial “company” produce numerous compounds that profoundly have an effect on the best way we digest meals, make the most of vitality, and assist our immunity, amongst many different capabilities. Regardless of the significance of our microbiome for our well being, to this point there was no silver bullet to stop antibiotic-induced dysbiosis.
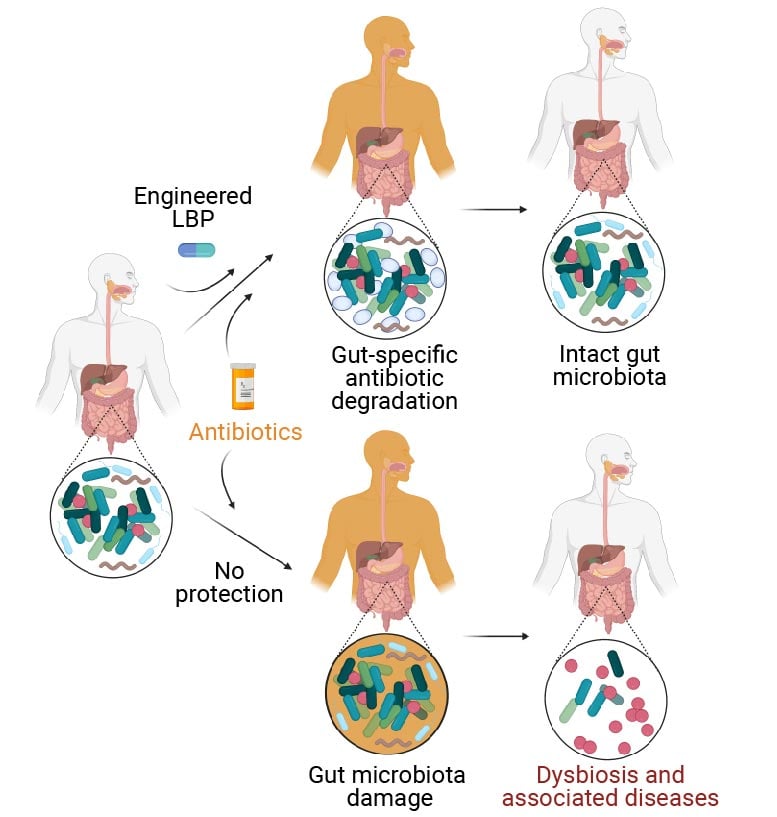
Human sufferers handled with antibiotics endure harm to their intestinal microbiome (decrease department) which causes its regular composition to be thrown off-balance. Often known as “dysbiosis,” this imbalance is a threat issue for a number of autoimmune, metabolic, and neurological illnesses. As an answer, treating sufferers that obtain an antibiotic concurrently with an engineered reside biotherapeutic product (eLBP, higher department) developed on the Wyss Institute and MIT, which degrades antibiotics within the intestine, prevents the harmful results of antibiotics on the intestinal microbiome. Because the eLBP doesn't change the focus of antibiotics within the blood, they nonetheless attain infections in every single place else within the physique. Credit score: Wyss Institute at Harvard College
Now, a analysis workforce on the Wyss Institute for Biologically Impressed Engineering at Harvard College and Massachusetts Institute of Expertise (MIT), utilizing an artificial biology strategy, has developed an engineered reside biotherapeutic product (eLBP) that, when given along with generally used antibiotics often called ß-lactams (which incorporates the well-known antibiotic penicillin), protects the intestine microbiome from dysbiosis. The research is revealed in Nature Biomedical Engineering.
“In designing the eLBP, we tapped into the artificial biology took package that now we have superior over the previous twenty years and enabled Lactococcus lactis, a safe-to-use microbe, to secrete a ß-lactamase enzyme that altruistically degrades ß-lactams within the micro organism’s setting,” stated Wyss Core College member and lead of the Institute’s Residing Mobile System Platform, James Collins, Ph.D., who led the research. “The enzyme primarily turns into a ‘widespread good’ that can't confer a selective benefit to the manufacturing micro organism or be simply transferred to different micro organism, minimizing the danger and maximizing the scientific advantages of our strategy.” Collins can also be the Termeer Professor of Medical Engineering & Science at MIT. In 2018, his workforce used L. lactis to develop an engineered probiotic intervention to detect and deal with cholera infections.
Safety from antibiotics goes reside
Often, ß-lactamase enzymes are encoded by a single gene that may be handed between micro organism through a course of known as horizontal gene switch, and the enzymes themselves reside throughout the micro organism’s cell wall or membrane enclosures. This not solely makes the manufacturing bacterial pressure proof against sure antibiotics that assault these outer enclosures, but additionally permits their resistance to unfold to different micro organism within the intestine microbial inhabitants. “To safeguard in opposition to the event and unfold of antibiotic resistance, we engineered numerous management models into the ß-lactamase expression system. Primarily, we break up a selected ß-lactamase-encoding gene, distributed the 2 genetically unlinked halves to completely different elements of the bacterium’s DNA, and additional engineered them in order that they'd be secreted away from the producer cell and bind to one another with excessive affinity to reassemble a practical enzyme in its outdoors setting,” stated first-author Andrés Cubillos-Ruiz, Ph.D., who spearheaded the venture in Collins’ group.
Cubillos-Ruiz and his co-workers on Collins’ workforce then confirmed that once they gave their eLBP intervention to mice that acquired the antibiotic ampicillin as an oral remedy, it certainly minimized dysbiosis in every animal’s intestine. By sequencing part of the bacterial genome often called 16S rDNA, which gives a genetic zip code for all bacterial species and households, they discovered that the eLBP considerably dampened the collapse of microbial populations and allowed them to totally recuperate their unique range and composition three days after antibiotic remedy. Mice handled with ampicillin that weren't protected by the eLPB, suffered a a lot larger lack of their microbial range which they may not recuperate throughout your complete course of the experiment.
“Importantly, throughout its transient keep within the digestive tract, the eLBP protected the microbiome with out altering the focus of ampicillin circulating within the blood, which is necessary as a result of the antibiotic nonetheless wants to achieve infections in every single place else within the physique to do its job,” stated Cubillos-Ruiz. “The eLBP additionally lowered the enrichment of assorted antibiotic resistance genes throughout the microbial neighborhood, which generally occurs below the selective strain of antibiotics.” The intestine microbiome accommodates a pure pool of micro organism with genes, together with ß-lactamase genes, that induce resistance to antibiotics by way of completely different mechanisms – together with resistance even to unrelated antibiotics. With each antibiotic remedy their numbers shoot up and in addition contribute to the unfold of antibiotic resistance by horizontal gene switch.
“We at the moment are specializing in getting these dwelling therapies to sufferers and are finalizing the design of an efficient, brief, and cheap scientific trial. We additionally imagine that our common eLBP strategy will be prolonged right into a therapeutic platform that could possibly be utilized not solely to different antibiotics, but additionally to handle illnesses the place intestine dysbiosis is on the middle.”
— Andrés Cubillos-Ruiz
Maintaining a lid on opportunistic micro organism
Lastly, the workforce addressed a standard consequence of dysbiosis: the hostile takeover of the vacated intestinal territory by problematic micro organism like Clostridioides difficile. These “opportunistic micro organism” reside in many individuals’s intestines at decrease numbers and, when given the prospect to multiply uncontrollably, set off irritation, diarrhea, and should contribute to the event of inflammatory bowel illness. The workforce modeled a full-fledged human C. difficile an infection by infecting ampicillin-treated mice with spores of C. difficile. The eLBP efficiently prevented the intestinal colonization with C. difficile, in distinction to a traditional unmodified L. lactis pressure not producing the break up ß-lactamase enzyme.
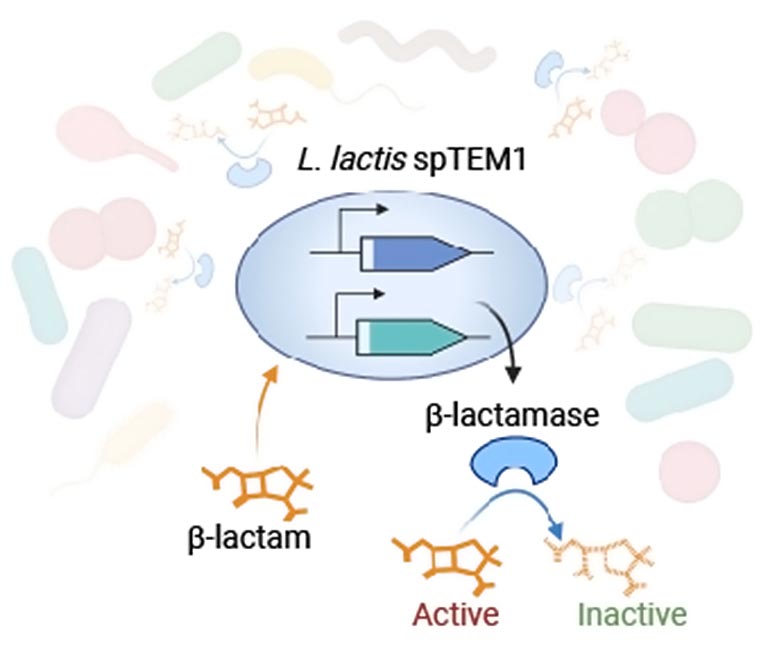
Utilizing artificial biology instruments, the workforce engineered the safe-to-use microbe Lactococcus lactis to secrete a β-lactamase enzyme known as TEM1 that altruistically degrades β-lactams within the micro organism’s setting. To make their strategy secure by stopping the manufacturing pressure from having a selective benefit over different strains within the intestine, and spreading a totally practical TEM1 gene by way of the intestine microbial neighborhood through “horizontal gene switch,” they break up the enzyme-encoding gene, and distributed each halves to completely different elements of the micro organism’s DNA. Translated into protein and secreted into the micro organism’s setting, they reassemble a practical β-lactamase enzyme that inactivates β-lactam antibiotics. Credit score: Wyss Institute at Harvard College
“This is among the strongest examples of an engineered dwelling mobile remedy tackling a urgent scientific downside popping out of academia to this point,” stated Collins.
“We at the moment are specializing in getting these dwelling therapies to sufferers and are finalizing the design of an efficient, brief, and cheap scientific trial,”stated Cubillos-Ruiz, including that, “We additionally imagine that our common eLBP strategy will be prolonged right into a therapeutic platform that could possibly be utilized not solely to different antibiotics, but additionally to handle illnesses the place intestine dysbiosis is on the middle.”
This elegantly engineered and extremely efficient dwelling mobile therapeutic gadget might turn out to be a real sport changer within the remedy of infectious illnesses each by serving to to take care of a wholesome microbiome in sufferers handled with antibiotics and, maybe equally necessary within the longer run, by stopping the rising downside of antibiotic resistance which is a rising downside worldwide,” stated Wyss Founding Director Donald Ingber, M.D., Ph.D., who can also be the Judah Folkman Professor of Vascular Biology at HMS and Boston Youngsters’s Hospital, and Hansjörg Professor of Bioinspired Engineering on the Harvard John A. Paulson Faculty of Engineering and Utilized Sciences.
Reference: “An engineered reside biotherapeutic for the prevention of antibiotic-induced dysbiosis” by Andrés Cubillos-Ruiz, Miguel A. Alcantar, Nina M. Donghia, Pablo Cárdenas, Julian Avila-Pacheco and James J. Collins, 11 April 2022, Nature Biomedical Engineering.
DOI: 10.1038/s41551-022-00871-9
Different authors on the research are Miguel Alcantar and Nina Donghia from Collins’ group, Pablo Cárdenas from the MIT Division of Organic Engineering, and Julian Avila-Pacheco from the Broad Institute. The research was funded by the Wyss Institute at Harvard College, a Protection Menace Discount Company grant (#HDTRA1-14-1-0006), and the Paul G. Allen Frontiers Group.
Post a Comment