Yogesh Surendranath and his group are bringing highly effective strategies of electrochemistry to bear on the issue of designing catalysts for sustainable fuels.
One problem in decarbonizing the vitality system is figuring out methods to cope with new varieties of fuels. Conventional fuels comparable to pure fuel and oil may be mixed with different supplies after which heated to excessive temperatures in order that they chemically react to supply different helpful fuels or substances, and even vitality to do work. However new supplies comparable to biofuels can’t take as a lot warmth with out breaking down.
A key ingredient in such chemical reactions is a specifically designed strong catalyst that's added to encourage the response to occur however isn’t itself consumed within the course of. With conventional supplies, the strong catalyst usually interacts with a fuel; however with fuels derived from biomass, for instance, the catalyst should work with a liquid — a particular problem for many who design catalysts.
For practically a decade, Yogesh Surendranath, an affiliate professor of chemistry at MIT, has been specializing in chemical reactions between strong catalysts and liquids, however in a distinct state of affairs: moderately than utilizing warmth to drive reactions, he and his group enter electrical energy from a battery or a renewable supply comparable to wind or photo voltaic to present chemically inactive molecules extra vitality in order that they react. And key to their analysis is designing and fabricating strong catalysts that work properly for reactions involving liquids.
Recognizing the necessity to use biomass to develop sustainable liquid fuels, Surendranath puzzled whether or not he and his group may take the rules they've realized about designing catalysts to drive liquid-solid reactions with electrical energy and apply them to reactions that happen at liquid-solid interfaces with none enter of electrical energy.
To their shock, they discovered that their data is straight related. Why? “What we discovered — amazingly — is that even once you don’t hook up wires to your catalyst, there are tiny inside ‘wires’ that do the response,” says Surendranath. “So, reactions that folks usually suppose function with none movement of present really do contain electrons shuttling from one place to a different.” And that implies that Surendranath and his group can deliver the highly effective strategies of electrochemistry to bear on the issue of designing catalysts for sustainable fuels.
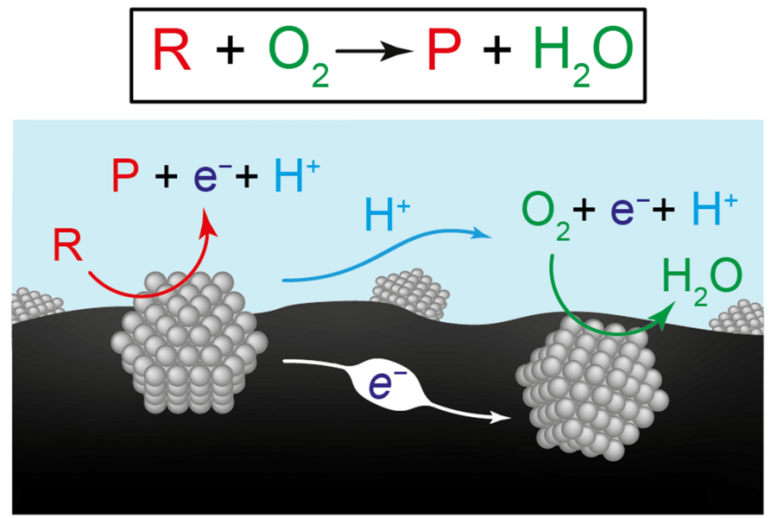
This determine presents two views of the chemical reactions for producing renewable fuels and chemical compounds. The highest equation represents the conversion of the reactant (R) plus oxygen (O2) to a product (P) plus water (H2O). The diagram under illustrates researchers’ speculation that the general response is the results of two coordinated half-reactions occurring on separate catalyst supplies, right here represented by grey constructions. On the left-hand catalyst, the reactant turns right into a product, sending electrons (e-) into the carbon assist materials (black) and protons (H+) into water (blue). On the right-hand catalyst, electrons and protons are consumed as they drive the response of oxygen to water.
Credit score: Picture courtesy of the researchers.
A novel speculation
Their work has targeted on a category of chemical reactions necessary within the vitality transition that contain including oxygen to small natural (carbon-containing) molecules comparable to ethanol, methanol, and formic acid. The standard assumption is that the reactant and oxygen chemically react to kind the product plus water. And a strong catalyst — typically a mixture of metals — is current to supply websites on which the reactant and oxygen can work together.
However Surendranath proposed a distinct view of what’s happening. Within the common setup, two catalysts, every one composed of many nanoparticles, are mounted on a conductive carbon substrate and submerged in water. In that association, negatively charged electrons can movement simply via the carbon, whereas positively charged protons can movement simply via water.
Surendranath’s speculation was that the conversion of reactant to product progresses by way of two separate “half-reactions” on the 2 catalysts. On one catalyst, the reactant turns right into a product, within the course of sending electrons into the carbon substrate and protons into the water. These electrons and protons are picked up by the opposite catalyst, the place they drive the oxygen-to-water conversion. So, as a substitute of a single response, two separate however coordinated half-reactions collectively obtain the web conversion of reactant to product.
Because of this, the general response doesn’t really contain any internet electron manufacturing or consumption. It's a normal “thermal” response ensuing from the vitality within the molecules and perhaps some added warmth. The standard strategy to designing a catalyst for such a response would concentrate on rising the speed of that reactant-to-product conversion. And one of the best catalyst for that sort of response may transform, say, gold or palladium or another costly treasured steel.
Nevertheless, if that response really entails two half-reactions, as Surendranath proposed, there's a movement of electrical cost (the electrons and protons) between them. So Surendranath and others within the subject may as a substitute use strategies of electrochemistry to design not a single catalyst for the general response however moderately two separate catalysts — one to hurry up one half-reaction and one to hurry up the opposite half-reaction. “Meaning we don’t must design one catalyst to do all of the heavy lifting of rushing up your complete response,” says Surendranath. “We would be capable to pair up two low-cost, earth-abundant catalysts, every of which does half of the response properly, and collectively they perform the general transformation shortly and effectively.”
However there’s another consideration: Electrons can movement via your complete catalyst composite, which encompasses the catalyst particle(s) and the carbon substrate. For the chemical conversion to occur as shortly as doable, the speed at which electrons are put into the catalyst composite should precisely match the speed at which they're taken out. Specializing in simply the electrons, if the reaction-to-product conversion on the primary catalyst sends the identical variety of electrons per second into the “bathtub of electrons” within the catalyst composite because the oxygen-to-water conversion on the second catalyst takes out, the 2 half-reactions might be balanced, and the electron movement — and the speed of the mixed response — might be quick. The trick is to seek out good catalysts for every of the half-reactions which can be completely matched when it comes to electrons in and electrons out.
“An excellent catalyst or pair of catalysts can keep an electrical potential — primarily a voltage — at which each half-reactions are quick and are balanced,” says Jaeyune Ryu PhD ’21, a former member of the Surendranath lab and lead creator of the research; Ryu is now a postdoc at Harvard College. “The charges of the reactions are equal, and the voltage within the catalyst composite gained’t change throughout the general thermal response.”
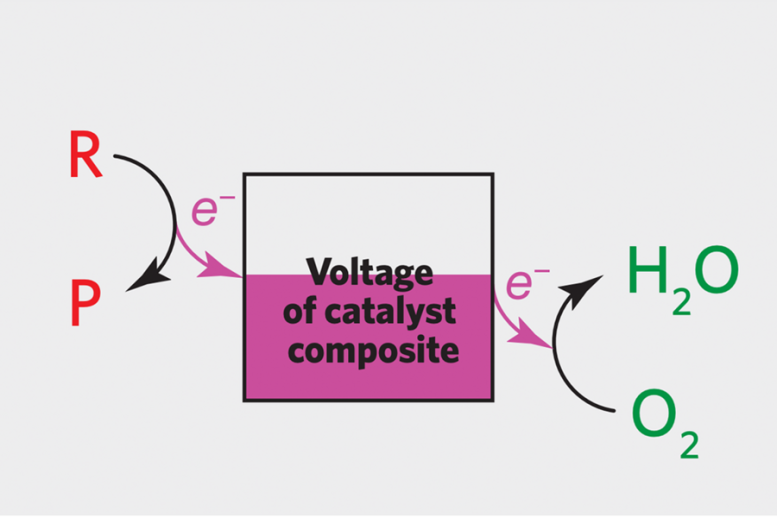
On this diagram, the 2 “hidden” half-reactions answerable for the noticed catalysis are depicted on reverse sides of a field during which the voltage stage of the catalyst composite (the catalysts plus the carbon substrate) is indicated as pink. The conversion of reactant to product is on the left, and the conversion of oxygen to water is on the precise. With a well-matched pair of catalysts, the response on the left will launch electrons on the similar charge because the response on the proper picks them up, and the voltage might be fixed. The aim is for that matching to happen when each response charges are excessive.
Credit score: Picture courtesy of the researchers.
Drawing on electrochemistry
Based mostly on their new understanding, Surendranath, Ryu, and their colleagues turned to electrochemistry strategies to determine catalyst for every half-reaction that may additionally pair as much as work properly collectively. Their analytical framework for guiding catalyst improvement for techniques that mix two half-reactions relies on a principle that has been used to grasp corrosion for nearly 100 years, however has hardly ever been utilized to grasp or design catalysts for reactions involving small molecules necessary for the vitality transition.
Key to their work is a potentiostat, a kind of voltmeter that may both passively measure the voltage of a system or actively change the voltage to trigger a response to happen. Of their experiments, Surendranath and his group use the potentiostat to measure the voltage of the catalyst in actual time, monitoring the way it adjustments millisecond to millisecond. They then correlate these voltage measurements with simultaneous however separate measurements of the general charge of catalysis to grasp the response pathway.
For his or her research of the conversion of small, energy-related molecules, they first examined a collection of catalysts to seek out good ones for every half-reaction — one to transform the reactant to product, producing electrons and protons, and one other to transform the oxygen to water, consuming electrons and protons. In every case, a promising candidate would yield a speedy response — that's, a quick movement of electrons and protons out or in.
To assist determine an efficient catalyst for performing the primary half-reaction, the researchers used their potentiostat to enter rigorously managed voltages and measured the ensuing present that flowed via the catalyst. An excellent catalyst will generate a lot of present for little utilized voltage; a poor catalyst would require excessive utilized voltage to get the identical quantity of present. The group then adopted the identical process to determine catalyst for the second half-reaction.
To expedite the general response, the researchers wanted to seek out two catalysts that matched properly — the place the quantity of present at a given utilized voltage was excessive for every of them, making certain that as one produced a speedy movement of electrons and protons, the opposite one consumed them on the similar charge.
To check promising pairs, the researchers used the potentiostat to measure the voltage of the catalyst composite throughout internet catalysis — not altering the voltage as earlier than, however now simply measuring it from tiny samples. In every take a look at, the voltage will naturally settle at a sure stage, and the aim is for that to occur when the speed of each reactions is excessive.
Validating their speculation and looking out forward
By testing the 2 half-reactions, the researchers may measure how the response charge for every one various with adjustments within the utilized voltage. From these measurements, they may predict the voltage at which the complete response would proceed quickest. Measurements of the complete response matched their predictions, supporting their speculation.
The group’s novel strategy of utilizing electrochemistry strategies to look at reactions regarded as strictly thermal in nature gives new insights into the detailed steps by which these reactions happen and subsequently into methods to design catalysts to hurry them up. “We are able to now use a divide-and-conquer technique,” says Ryu. “We all know that the web thermal response in our research occurs via two ‘hidden’ however coupled half-reactions, so we are able to goal to optimize one half-reaction at a time” — presumably utilizing low-cost catalyst supplies for one or each.
Provides Surendranath, “One of many issues that we’re enthusiastic about on this research is that the outcome will not be last in and of itself. It has actually seeded a brand-new thrust space in our analysis program, together with new methods to design catalysts for the manufacturing and transformation of renewable fuels and chemical compounds.”
This analysis was supported primarily by the Air Power Workplace of Scientific Analysis. Jaeyune Ryu PhD ’21 was supported by a Samsung Scholarship. Further assist was supplied by a Nationwide Science Basis Graduate Analysis Fellowship.
This text seems within the Autumn 2021 problem of Power Futures, the journal of the MIT Power Initiative.
Post a Comment