MIT researchers have recognized an issue that tends to restrict chemical processes for turning carbon dioxide into gasoline or different helpful chemical substances — and methods of addressing that drawback. Credit score: Courtesy of the Varanasi Lab
Examine reveals why some makes an attempt to transform the greenhouse fuel into gasoline have failed, and affords attainable options.
If researchers might discover a strategy to chemically convert carbon dioxide into fuels or different merchandise, they may make a serious dent in greenhouse fuel emissions. However many such processes which have appeared promising within the lab haven’t carried out as anticipated in scaled-up codecs that might be appropriate to be used with an influence plant or different emissions sources.
Now, researchers at MIT have recognized, quantified, and modeled a serious purpose for poor efficiency in such conversion programs. The wrongdoer seems to be a neighborhood depletion of the carbon dioxide fuel proper subsequent to the electrodes getting used to catalyze the conversion. The issue will be alleviated, the group discovered, by merely pulsing the present on and off at particular intervals, permitting time for the fuel to construct again as much as the wanted ranges subsequent to the electrode.
The findings, which might spur progress on creating a wide range of supplies and designs for electrochemical carbon dioxide conversion programs, have been printed on January 11, 2022, within the journal Langmuir, in a paper by MIT postdoc Álvaro Moreno Soto, graduate scholar Jack Lake, and professor of mechanical engineering Kripa Varanasi.
“Carbon dioxide mitigation is, I feel, one of many essential challenges of our time,” Varanasi says. Whereas a lot of the analysis within the space has centered on carbon seize and sequestration, during which the fuel is pumped into some type of deep underground reservoir or transformed to an inert stable resembling limestone, one other promising avenue has been changing the fuel into different carbon compounds resembling methane or ethanol, for use as gasoline, or ethylene, which serves as a precursor to helpful polymers.
There are a number of methods to do such conversions, together with electrochemical, thermocatalytic, photothermal, or photochemical processes. “Every of those has issues or challenges,” Varanasi says. The thermal processes require very excessive temperature, they usually don’t produce very high-value chemical merchandise, which is a problem with the light-activated processes as effectively, he says. “Effectivity is all the time at play, all the time a difficulty.”
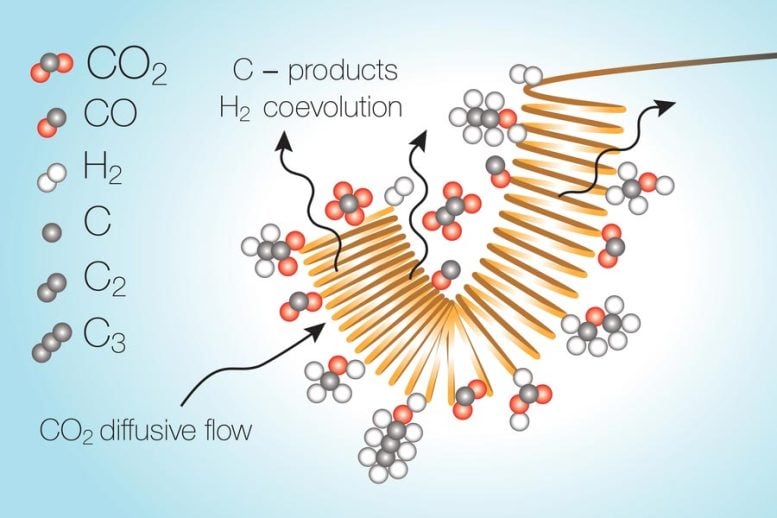
The group has centered on the electrochemical approaches, with a purpose of getting “higher-C merchandise” — compounds that include extra carbon atoms and are typically higher-value fuels due to their power per weight or quantity. In these reactions, the largest problem has been curbing competing reactions that may happen on the identical time, particularly the splitting of water molecules into oxygen and hydrogen.
The reactions happen as a stream of liquid electrolyte with the carbon dioxide dissolved in it passes over a steel catalytic floor that's electrically charged. However because the carbon dioxide will get transformed, it leaves behind a area within the electrolyte stream the place it has primarily been used up, and so the response inside this depleted zone turns towards water splitting as an alternative. This undesirable response makes use of up power and enormously reduces the general effectivity of the conversion course of, the researchers discovered.
“There’s quite a few teams engaged on this, and quite a few catalysts which might be on the market,” Varanasi says. “In all of those, I feel the hydrogen co-evolution turns into a bottleneck.”
A technique of counteracting this depletion, they discovered, will be achieved by a pulsed system — a cycle of merely turning off the voltage, stopping the response and giving the carbon dioxide time to unfold again into the depleted zone and attain usable ranges once more, after which resuming the response.
Typically, the researchers say, teams have discovered promising catalyst supplies however haven’t run their lab checks lengthy sufficient to look at these depletion results, and thus have been annoyed in making an attempt to scale up their programs. Moreover, the focus of carbon dioxide subsequent to the catalyst dictates the merchandise which might be made. Therefore, depletion also can change the combo of merchandise which might be produced and may make the method unreliable. “If you'd like to have the ability to make a system that works at industrial scale, you want to have the ability to run issues over a protracted time frame,” Varanasi says, “and it's essential not have these sorts of results that cut back the effectivity or reliability of the method.”
The group studied three totally different catalyst supplies, together with copper, and “we actually centered on ensuring that we understood and may quantify the depletion results,” Lake says. Within the course of they have been capable of develop a easy and dependable approach of monitoring the effectivity of the conversion course of because it occurs, by measuring the altering pH ranges, a measure of acidity, within the system’s electrolyte.
Of their checks, they used extra refined analytical instruments to characterize response merchandise, together with fuel chromatography for evaluation of the gaseous merchandise, and nuclear magnetic resonance characterization for the system’s liquid merchandise. However their evaluation confirmed that the straightforward pH measurement of the electrolyte subsequent to the electrode throughout operation might present a enough measure of the effectivity of the response because it progressed.
Now that the method is known and quantified, different approaches to mitigating the carbon dioxide depletion could be developed, the researchers say, and will simply be examined utilizing their strategies.
This work exhibits, Lake says, that “it doesn't matter what your catalyst materials is” in such an electrocatalytic system, “you’ll be affected by this drawback.” And now, through the use of the mannequin they developed, it’s attainable to find out precisely what sort of time window must be evaluated to get an correct sense of the fabric’s general effectivity and what sort of system operations might maximize its effectiveness.
Reference: “Transient Results Brought on by Gasoline Depletion throughout Carbon Dioxide Electroreduction” by Álvaro Moreno Soto, Jack R. Lake and Kripa Ok. Varanasi, 11 January, Langmuir.
DOI: 10.1021/acs.langmuir.1c02540
The analysis was supported by Shell, via the MIT Power Initiative.
Post a Comment